For millions of people every year, depression is not just an illness but a grueling pattern — anguish, meds, failure, repeat.
Supported by a major grant, two Harvard scientists want to break that pattern, each by his own path.
David Walt is operating at a microscopic level, observing cell abnormalities that may contribute to depression. Diego Pizzagalli is taking a bigger-picture approach, using MRIs and other methods to identify potential treatments by tracking activity in key brain regions. Their common aim, backed by the nonprofit Wellcome Leap, is to speed the path from diagnosis to an effective medication for the individual patient.
“We’re concerned that when people go through this trial-and-error approach, they lose hope,” Pizzagalli said. “We’re really interested in evaluating whether by using tools of neuroscience, we can get to the correct treatment faster.”
More than 22 million U.S. adults suffer at least one major depressive episode ever year. The experience is lonely, debilitating, and dangerous. As anxiety, insomnia, and other symptoms take hold, patients lose touch with family and friends. Feelings of isolation interrupt one of the greatest sources of happiness and well-being — relationships — and heighten their risk of suicide. The damage also creeps into broader society, including U.S. workplaces, imposing an economic burden of more than $330 billion annually.
The first attempt at antidepressant therapy takes 12-14 weeks to be effective, and works for only about a third of patients.
Research shows varying success with subsequent treatments, with as little as 40 percent of patients able to find a drug that works for them by the fourth try.

Talk therapy can help, and emerging technologies, including neurostimulation, have shown promise. But one of the most common treatments for depression — antidepressants such as selective serotonin reuptake inhibitors, or SSRIs, which are often prescribed by a primary care physician — has yielded mixed results, in part because of a grindingly inexact matching process.
“You end up with lots of people who, frankly, need a personalized individualized analysis to figure out the underlying basis of their disease that can be addressed by a particular drug,” Walt said. “It’s just sort of guesswork right now and there is no strong scientific basis for what’s right for each person. It’s, ‘Let’s try this drug and see if it works.’”
The goal of both researchers is to help shape an approach that is more effective for being more precise. “We’ve wanted to convince ourselves — and the field, hopefully — that personalized treatment is possible in depression,” Pizzagalli said. “Persistent symptoms can be very impairing and failed antidepressant treatments are associated with costs to individuals and society, with loss of productivity.”
Written in the blood

David Walt in his lab at the Wyss Institute.
Photo by Niles Singer
Walt, a professor of pathology and the Hansjörg Wyss Professor of Bioinspired Engineering at Harvard Medical School, wants to know whether certain proteins at work in the brain can shed light on how depression develops, allowing scientists to identify potential treatments. He and his team are studying four major cell types, each serving a different function with unique protein molecules.
“The expectation is that the proteins that we capture and measure from these four different cells will be different in individuals who suffer from major depression compared to those from healthy individuals,” Walt said.
One target is neurons, which transmit messages among brain regions. Changes in neurotransmitters such as serotonin can contribute to depression. (SSRIs function by increasing serotonin levels in the brain.) The other areas of focus are oligodendrocytes, microglia, and astrocytes, which influence cell structure, immune response, and metabolic function, respectively.
Any abnormality in these cells can weaken the connections in the brain and leave a person more vulnerable to mood disorders. Past research has suggested that antidepressants can help our brains repair and form new connections among damaged cells.
If investigators can determine which cell types are being affected in patients with depression, then eventually they should be able to target the underlying mechanism responsible for those changes, Walt said. If it’s a neurotransmission problem, then specialists can focus on finding drugs, including SSRIs, that best target neuron growth and regulation. If the issue is related to immune cells, researchers can try to identify drugs that affect the immune system.
Walt has zeroed in on extracellular vesicles — pieces of cells that travel out of the brain and into our blood.
“Parts of the cell membrane that encapsulate the cell break off from the cell into these really tiny nanoparticles,” he said. “These nanoparticles contain all the contents of the cell from which they have broken off. And these nanoparticles can get through the blood-brain barrier into the bloodstream to some extent.”
By comparing blood — which contains less than 1 percent material from the brain — with spinal fluid, he and his team have been able to identify specific markers in these different cell types that allow them to isolate extracellular vesicles in the blood.
The goal he has in mind would be life-changing.
“If you could identify the right markers in blood, then you could give a drug to somebody, and then have them come back the next week, take their blood, and measure biomarkers to determine if the drug is working,” he said.
“You may say, ‘This isn’t working, because your markers are exactly where they were last week before you started taking the drug. We need to switch you to a new drug immediately.’ Our goal is to avoid having patients wait six months to see if a drug works. If we can, it means we’re making progress toward helping these patients find the right treatment, compress the timeframe, and reduce the risk of suicide.”
Watching for a breakthrough

Diego Pizzagalli prepares a research participant for an EEG at McLean Hospital.
Photo by Niles Singer
Pizzagalli, a psychiatry professor at the Medical School and director of the Center for Depression, Anxiety and Stress Research at McLean Hospital, has spent his career examining psychological, environmental, and neurobiological factors associated with mood disorders, including major depression.
For the Wellcome Leap project, his lab is probing behavior and brain function for markers that could be used to assess the severity of a patient’s depression and guide treatment choices.
The work builds on a previous study that deployed neurocognitive tests, EEG, and functional MRI to pinpoint biomarkers that could predict a positive response to widely prescribed drugs: the atypical antidepressant bupropion, whose brand name is Wellbutrin, or the SSRI sertaline, whose brand name is Zoloft. That research led the team to imaging methods that in both cases reliably predicted a favorable response. The working premise now is that an MRI might be able to determine whether an SSRI or other medication is the best avenue of treatment.
“Our hope is that individuals with the bupropion markers will do very well when receiving bupropion, and vice versa for the patients with the sertraline markers,” said Pizzagalli, whose team will also weigh personal attributes (age, race, sex, etc.), personality traits, and performance in neuropsychological tests.
The functional MRI is recorded with patients in a resting state. Researchers track activated brain regions.
“Brain regions become activated with anything that we do: Thoughts, emotions, motivation, and so on,” said Pizzagalli, adding: “It’s not the case that every single brain region is activating in isolation and not communicating with the other brain regions. Information is basically passed from region to region.”
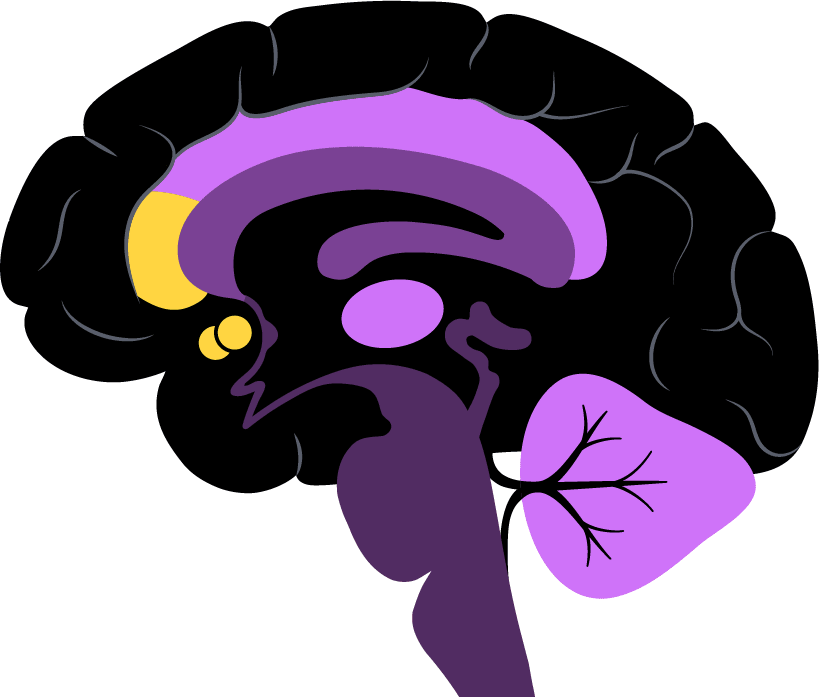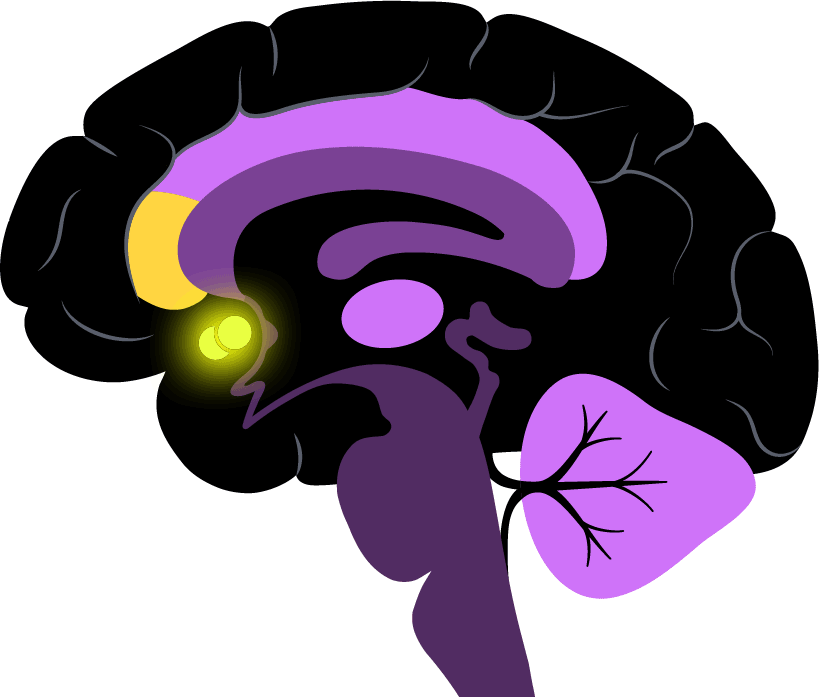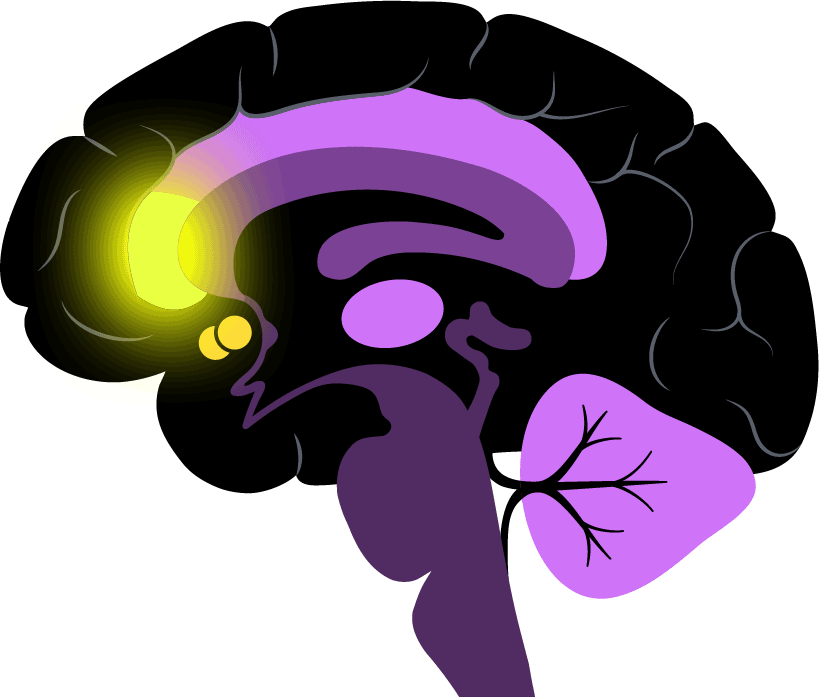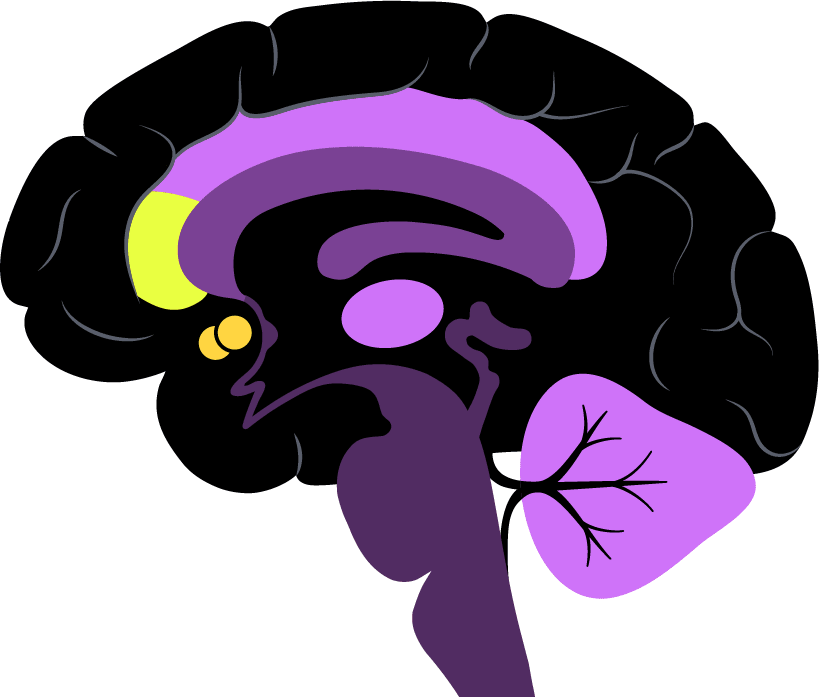
The two regions his team is most interested in are part of the so-called brain reward system. The nucleus accumbens is very deep in the brain and known for its role in pleasure and motivation; the rostral anterior cingulate cortex is located in the frontal lobe and a key intersection of cognition and emotion.
Pizzagalli is exploring the strength of the link between the two regions, which could help a prescriber decide between an SSRI and a non-SSRI.
“What we’re doing is moving from looking at the level of brain activity in a single region to activity across a network,” he said.
Both Walt and Pizzagalli pointed out that personalized treatment of depression and other brain disorders has been a challenge for reasons that go far beyond the capabilities of any one lab. Cost is a major obstacle, as is the deep individual complexity of the illness. But clarifying that such personalized treatment is possible and worth pursuing would be a major turning point, both for clinicians and their patients.
“The task is on researchers just to show whether these types of approaches can actually dramatically improve the response rate,” Pizzagalli said.
The journey to an answer is in its very early stages, both he and Walt were quick to note. Pizzagalli’s lab expects to finish work on their project in mid-to-late 2025. The first phase of Walt’s initiative is set to wrap this month, but the larger plan will unfold over years.
In the end, the researchers hope to have made major progress toward reclaiming time for patients and families suffering under the sometimes crushing weight of depression.
“It might be a blood test, it might be a blood test combined with imaging, it could be a blood test combined with imaging combined with certain behavioral features,” Walt said. “It could be that all of these tools, or a combination, will be necessary to really do precision diagnostics and be able to identify the right drug for the right person at the right time.”
If you or someone you know is struggling with a mental health issue, the National Institute of Mental Health has resources that can help. In a crisis, use the 988 Suicide and Crisis Lifeline. On campus, help is available through Counseling and Mental Health Services. There is also a 24/7 support line: 617-495-2042.
Source link

